Abstract
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer, accounting for 30% of pediatric cancers and 75% of pediatric leukemias. While overall survival in children with ALL exceeds 85%, survival rates for children who relapse are low. In recent years, targeted immunotherapy has shown significant responses in refractory ALL. CAR-modified T-cells and Blinatumomab are two immunotherapy treatments targeting the CD19 antigen that have shown to be effective in treating patients with relapse refractory ALL. Moxetumomab and Inotuzumab are agents that target CD22 on lymphoblasts. However, some patients do not respond and others relapse with antigen-negative disease after targeted therapy. Expression levels of CD19 and CD22 may predict response to therapy. Hence, it is important to quantify expression levels of CD19 and CD22 in B lymphoblastic leukemias and correlate with response to immunotherapy.
Methods
The flow cytometry archives at the Children's Hospital of Philadelphia from 2002 - 2017 were reviewed. The majority of cases reviewed were de-novo ALL and prior to any targeted immunotherapy. The flow cytometry panels included CD45-PC7, CD19-PE, CD10-PC5, CD9-FITC, CD20-ECD, CD38-FITC, CD34-ECD, and CD22-PE antibodies (all from BD Biosciences). During this time period, the flow cytometry panels were similar, run on similar instruments and analyzed the same way. To quantify the expression of CD19, a 4-point logarithmic scale was used. The scale ranged from 0-3 with 0 representing no expression, 1 representing dim expression, 2 representing normal expression in circulating B cells, and 3 representing bright expression of the surface antigen. CD19 vs CD10 dot plots of CD45dim gated pure populations of blasts were used to accurately quantify the number of CD19-negative events. Other antigens expressed on blasts such as CD34, CD24, CD9, CD38, and HLA-Dr were used if the blasts were CD10-negative. Surface CD22 expression was determined using a 3 point scale since CD22 expression is universally weak compared to CD19. Proportion of CD22 negative cells could not be determined as there was no other blast marker along with CD22 in our panel. Cytogenetics, FISH, and whole genome SNP array (2008-2017) data were obtained on all cases. All cases showed strong expression of CD79a, terminal deoxy transferase (TdT) and lacked strong myeloperoxidase (MPO) or CD3 expression ruling out AML or a mixed phenotype acute leukemia per the current WHO criteria. In parallel, RNA expression data from the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) study for Pediatric ALL was analyzed to identify cases with dim CD19 and CD22 expression.
Results
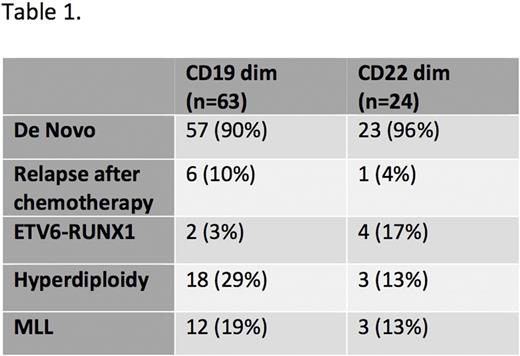
348 cases of B -ALL were identified, of which 304 were de novo and 45 were relapsed cases. 18% of all B-ALL specimens showed significant (>1%) populations of CD19-negative blasts. 7% of B-ALL specimens were negative for surface CD22. 5% of cases showed downregulation of both CD19 and CD22 antigens suggesting a correlation between various B cell antigens. Of the specimens that showed downregulation of CD19, 3% had the ETV6-RUNX1 translocation, 29% showed hyperdiploidy, and 19% showed rearrangement of the MLL gene. Of the specimens that showed downregulation of CD22, 17% showed the ETV6-RUNX1 translocation, 13% showed hyperdiploidy, and 13% showed rearrangement of the MLL gene. Cases with bright expression of CD19 or CD22 antigen were also determined. 12% of B-ALL showed overexpression of CD19 antigen when compared to normal mature B cells. 11% of B-ALL showed an overexpression of CD22 antigen when compared to normal mature B cells. Of the 157 cases analyzed from the TARGET study, 18% showed dim CD19 RNA expression and 11% showed dim CD22 RNA expression similar to the immunophenotypic findings.
Conclusion
We have comprehensively analyzed CD19 and CD22 expression in a large number of B-ALL cases from a single institution. We conclude that a significant number of B-ALL cases have dim CD19 and CD22 that may correlate with response to immunotherapy. Many cases also showed significant populations of CD19-negative cells that may be responsible for antigen negative relapses. We also identified cases with bright CD19 and CD22 that may predict good response to immunotherapy. Future directions include correlation of antigen expression with response to immunotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal